Introduction:
Five year overall survival for acute myeloid leukemia (AML) is estimated to be less than 30%. Encouraging results seen with the tyrosine kinase inhibitors (TKIs), midostaurin and gilteritinib resulted in the approval of these molecular targeted therapies for patients with FLT3 Mutated AML. Other TKIs like sorafenib and quizartinib, have ongoing clinical trials. In this systematic review and meta-analysis, we assessed the efficacy and safety of TKIs for the treatment of newly diagnosed (ND) and relapsed refractory (R/R) AML.
Methods:
A search was performed on PubMed, Cochrane, Embase, and clinicaltrials.gov. We used the keywords "tyrosine kinase inhibitors" AND "acute myeloid leukemia" from the inception of literature till 07/10/2020. We screened 3245 articles and included 5 randomized clinical trials (RCTs) (N=1919) in this meta-analysis. We extracted data for efficacy (i-e, OS, CR, ORR, EFS) and safety (≥grade 3 treatment related adverse events (TRAE). We excluded case reports, case series, preclinical studies, review articles, meta-analysis, observational studies, and controlled clinical studies not providing any information about the efficacy or safety of TKI. We used the R programming language (version 4.0.2) to conduct a meta-analysis.
Results:
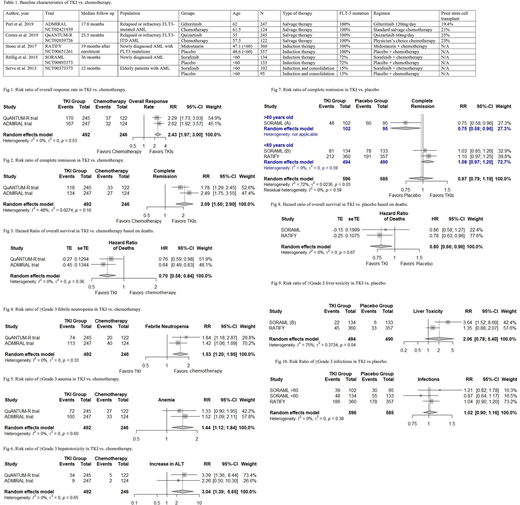
In the 5 RCTs (n=1919), the age range was 18-85 years. 1675 participants had FLT-3 mutation (Table 1). In 2 RCTs (N=738), two TKIs (gilteritinib and quizartinib) (N=492) were compared with salvage chemotherapy (N=246). Risk ratio (RR) of overall response rate (ORR) and complete remission (CR) was 2.43 (95% CI=1.97-3.00, I2=0) and 2.09 (95% CI=1.5-2.90, I2=48%), respectively in favor of TKIs. The hazard ratio (HR) for overall survival (OS) was 0.70 (95% CI=0.58-0.84, I2=0) in favor of TKIs. (Fig 1-3). The median OS was 6.2 months in the quizartinib group vs. 4.7 months in the chemotherapy group. Similarly, median OS was 9.3 months in the gilteritinib group vs. 5.6 months in the chemotherapy group. Grade 3 or higher TRAEs (anemia, infections, sepsis, febrile neutropenia, and liver toxicity) were reported more often in the TKI group vs. salvage chemotherapy group. (Fig 4-6).
In 3 RCTs (N=1181), two TKIs (midostaurin and sorafenib) (N=597) were compared with placebo (N=582). In the RCT evaluating role of sorafenib in older patients (>60 years) (N=197), RR of CR was 0.75 (95% CI=0.58-0.96) in favor of placebo. Although more patients died in the sorafenib group than the placebo group (23 vs 10 within 60-day period), TRAEs were similar in the two groups. In the remaining 2 RCTs, sorafenib and midostaurin were compared with placebo in younger patients (<60 years old) (N=982). RR of CR was 1.07 (95% CI=0.96-1.19, I2=0) in favor of TKIs and the hazard ratio for OS was 0.80 (95% CI=0.66-0.96, I2=0) in favor of TKIs (Fig 7,8). Median event-free survival (EFS) was 21 months in the sorafenib group vs. 9 months in the placebo group. Similarly, median EFS was 8.2 months in the midostaurin group vs. 3 months in the placebo group. Grade 3 or higher TRAEs (anemia, liver toxicity, infections, and diarrhea) were more common in the TKI group as compared to the placebo group. (Fig 9,10)
Conclusion:
Gilteritinib and quizartinib were not only better tolerated but also more effective than salvage chemotherapy in patients with FLT-3 mutated AML. In older patients, sorafenib appeared to have lower efficacy and higher toxicity when compared with placebo. In contrast, for younger patients, sorafenib and midostaurin had better efficacy and lower toxicity than placebo. Additional multicenter double-blind randomized clinical trials are needed to confirm these results.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.:Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal